top of page
mass
'no magic to the mole'
amount
molar mass
concentration
solution volume
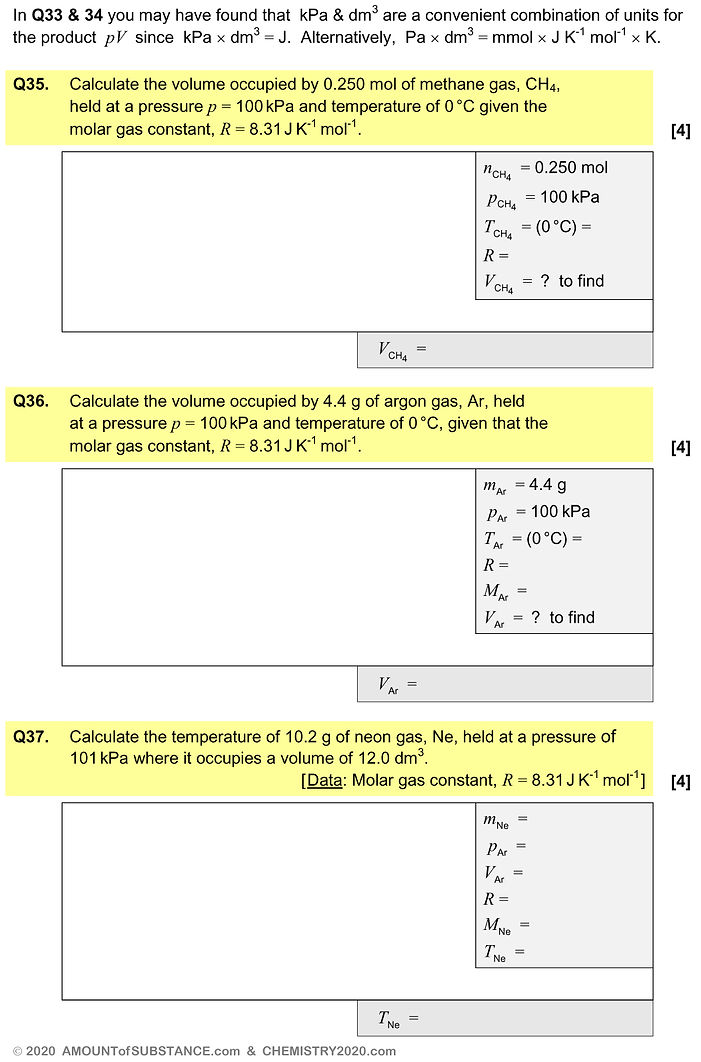
gas volume
molar gas volume
Avogadro
constant, L
number of
entities, N
Now try the following question.
One of the most important applications of the ideal gas equation is in the determination of molar masses of gases and volatile liquids. Two useful relationships are derived below from three equations that the reader will be familiar with already.

Instead of providing values of pressure and volume, questions involving the ideal gas equation might give the pressure and density and demand calculation of molar mass. In this case we have to make use of the relation m = r V.
bottom of page